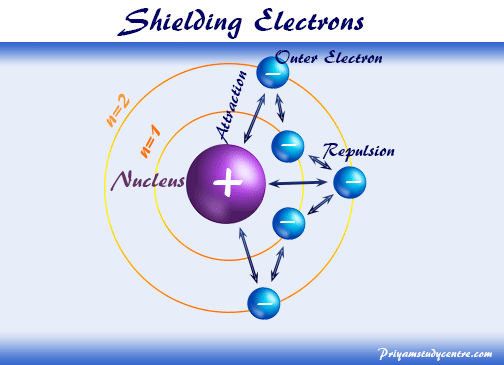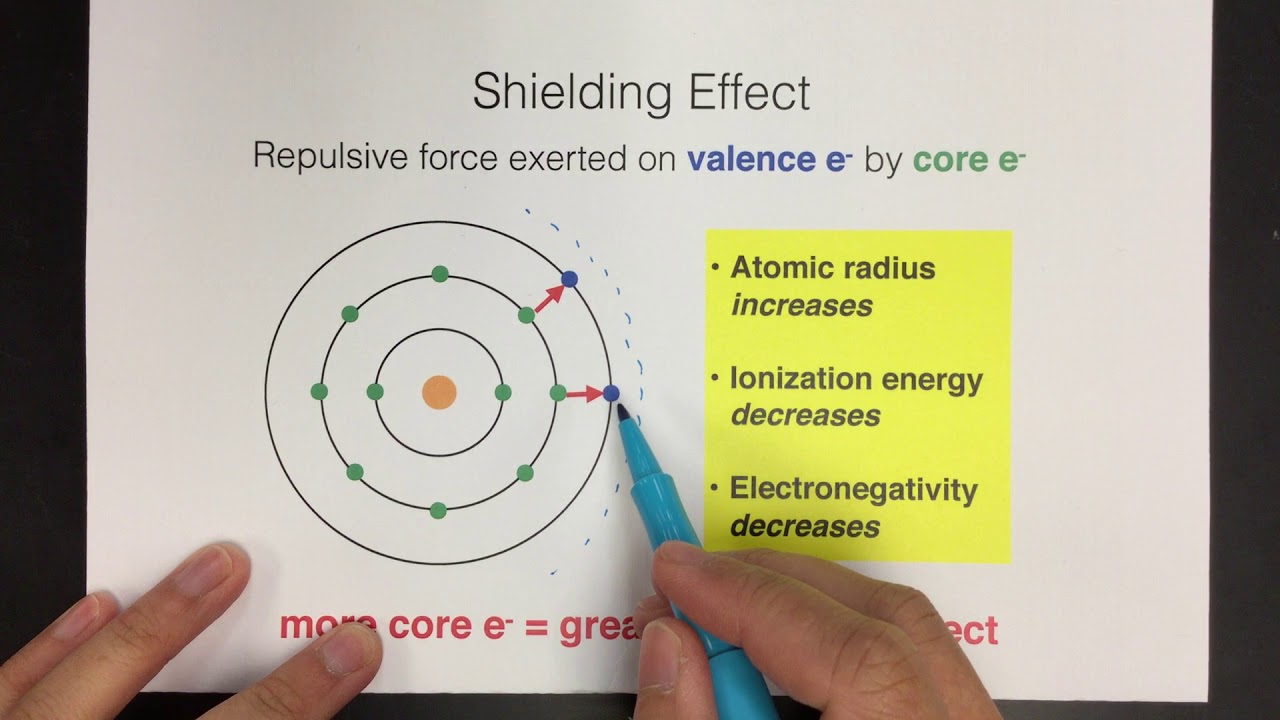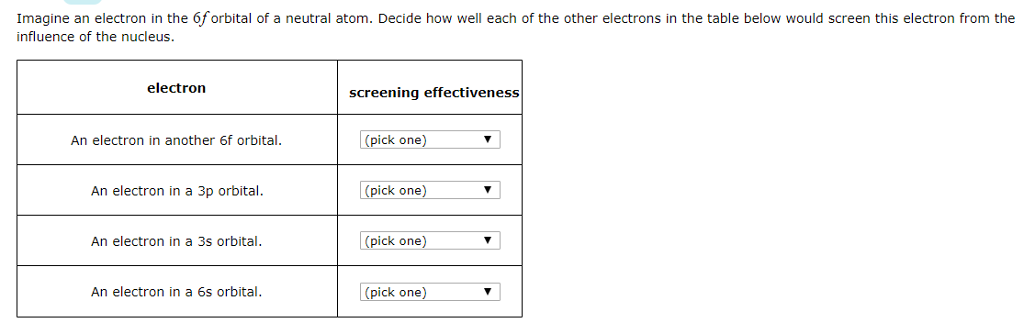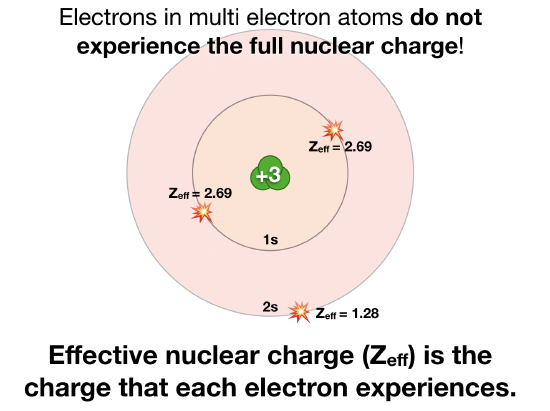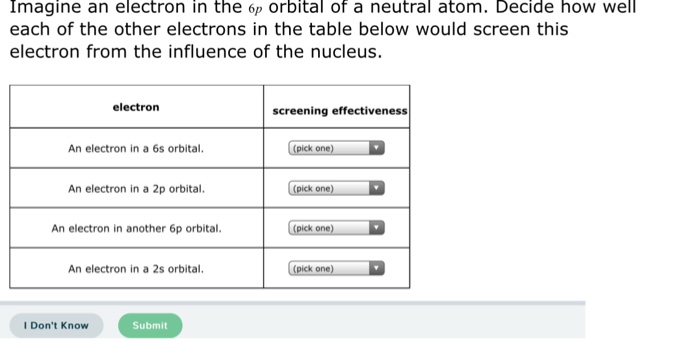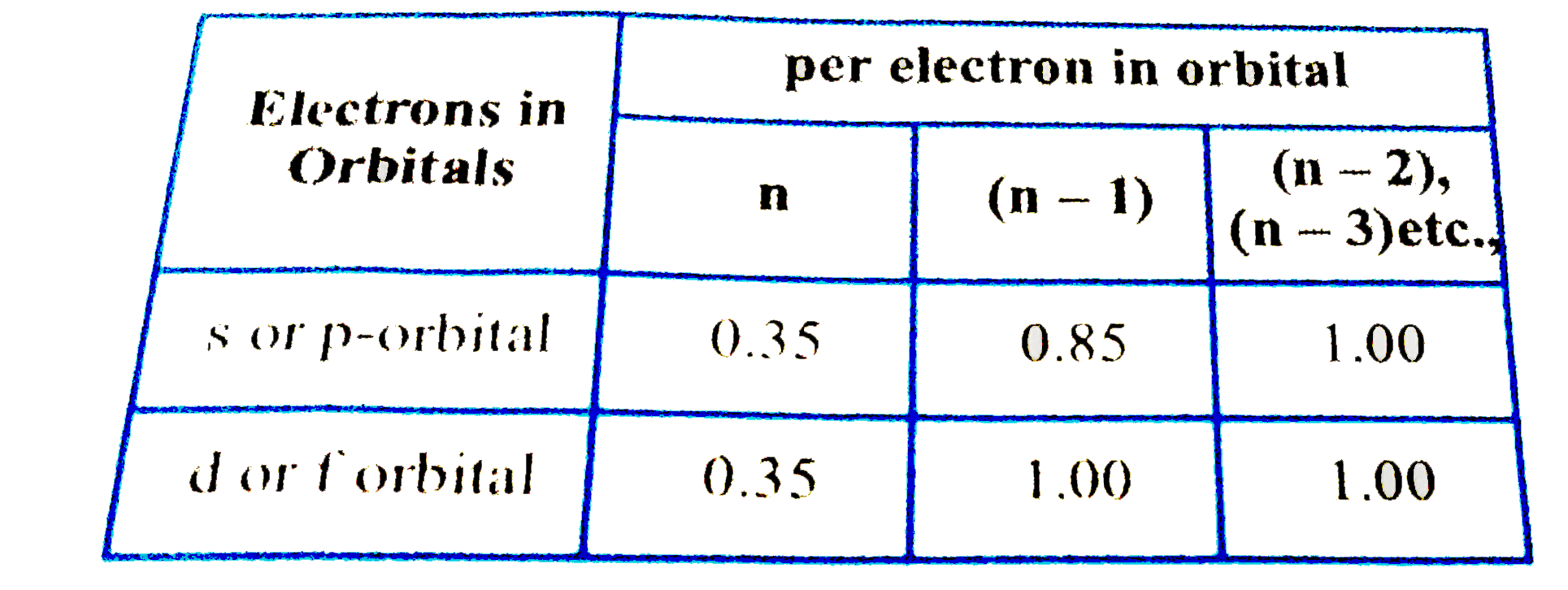
According to I.C slater effective nuclear charge, Z^(**), due to screening, is not exactly equal to the actual nuclear charge Z of the nucleus of the atom. Z^(**) depends on the type
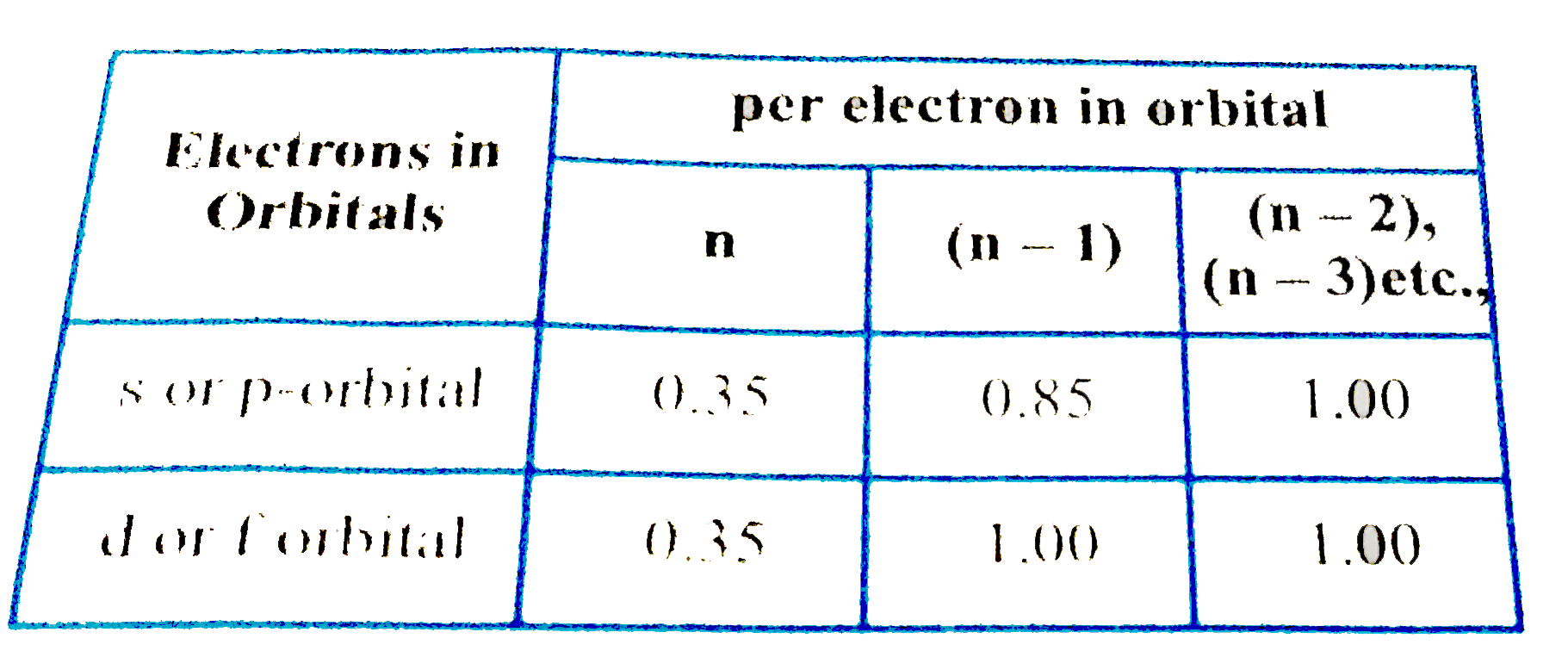
According to I.C slater effective nuclear charge, Z^(**), due to screening, is not exactly equal to the actual nuclear charge Z of the nucleus of the atom. Z^(**) depends on the type
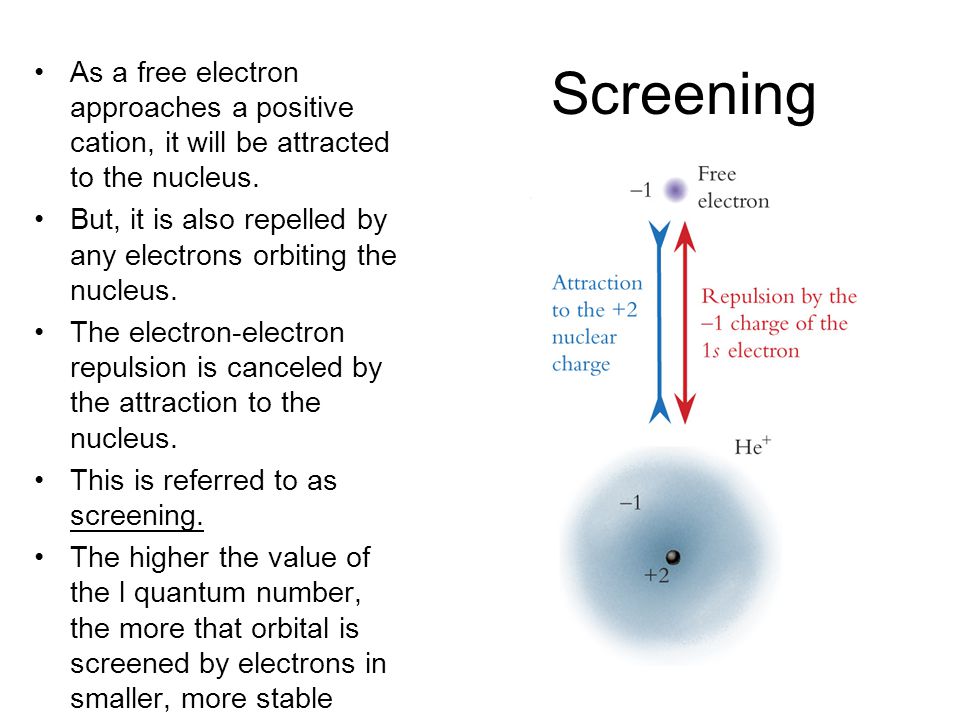
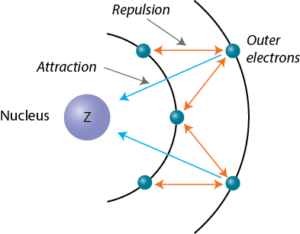
Screening As a free electron approaches a positive cation, it will be attracted to the nucleus. But, it is also repelled by any electrons orbiting the. - ppt download

PDF) Electron screening effect in the reactions 3He(d, p) 4He and d( 3He, p) 4He Supported in part by INFN, BMBF (06BO812), DFG (436UNG113-146) and OTKA (T025465 | Matthias Junker -
54. In the lithium atom screening effect of valence shell electron is caused by 1) Electron of k and L shell 2) Electron of k shell 3) Two electron of 1st and

Repulsive electron-electron interaction and nuclear charge screening: Ground state of two-electron atoms: Ndinya, Boniface, Akeyo, Joseph: 9783846540688: Amazon.com: Books